Product Information
Learn more about DefenCath, a catheter lock solution proven to reduce the incidence of CRBSIs.1
Find downloadable resources below.
Not Actual Size

DefenCath Prescribing Information
Download the Prescribing Information for DefenCath (taurolidine and heparin) catheter lock solution.
Download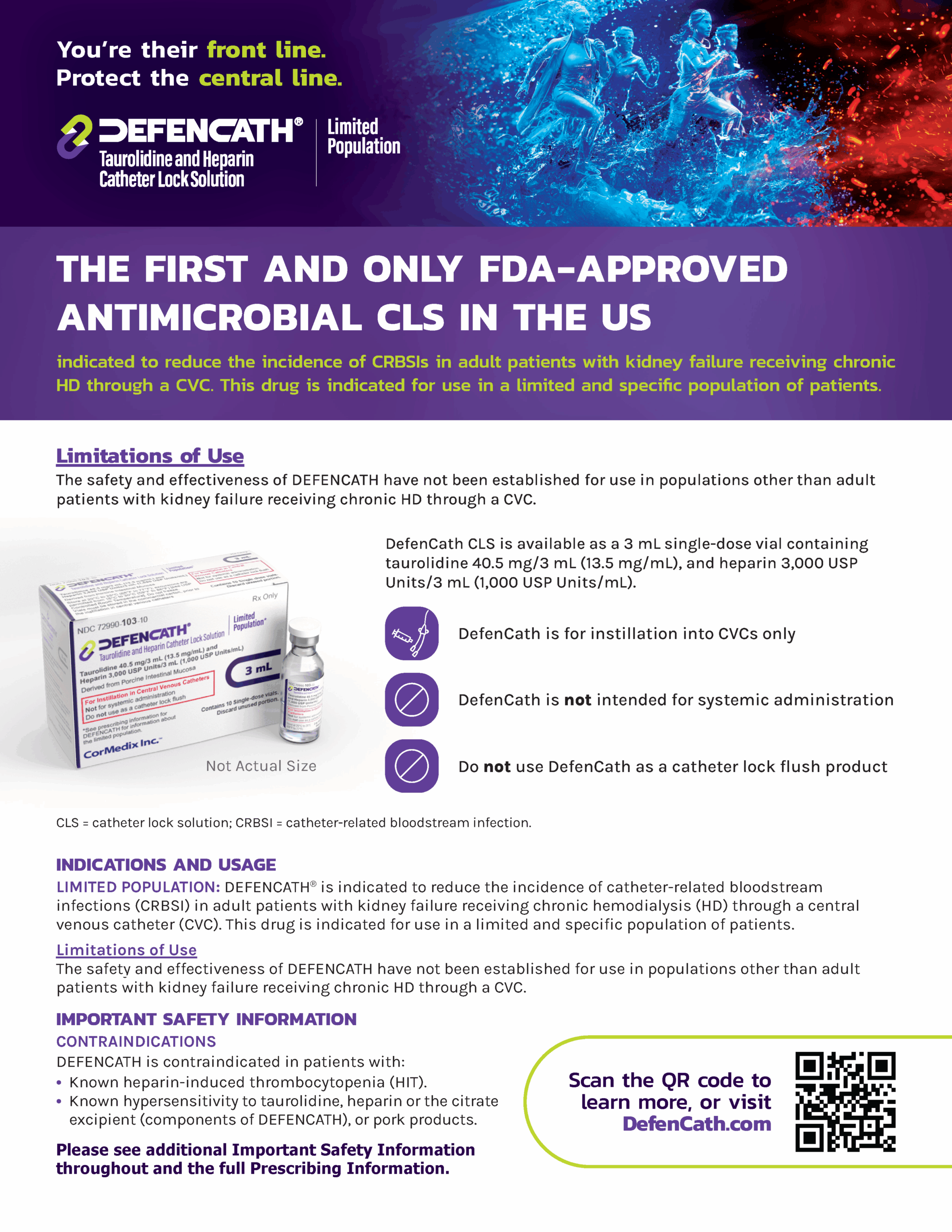
DefenCath Product Flyer
Download the Product Flyer for DefenCath (taurolidine and heparin) catheter lock solution.
Download
DefenCath Magnum Prescribing Information
Download the Prescribing Information for DefenCath (taurolidine and heparin) catheter lock solution.
Download
Agarwal A, et al. 2023 Clin J Am Soc Nephrol.
Access this study publication titled, “Taurolidine/Heparin Lock Solution and Catheter-Related Bloodstream Infection in Hemodialysis: A Randomized, Double-Blind, Active-Control, Phase 3 Study.”
Download
LOCK-IT-100 Overview
Download this digital brochure for a summary of the LOCK-IT-100 trial, which compared DefenCath (taurolidine and heparin) catheter lock solution to the current standard of care.
Download
Product Fact Sheet
Download this resource to learn more about DefenCath (taurolidine and heparin) catheter lock solution.
Download
Product Presentation
Download this presentation to learn more about DefenCath (taurolidine and heparin) catheter lock solution.
Download
FAQ Resource
Download this resource for details on frequently asked questions related to CRBSI’s and DefenCath (taurolidine and heparin) catheter lock solution.
Download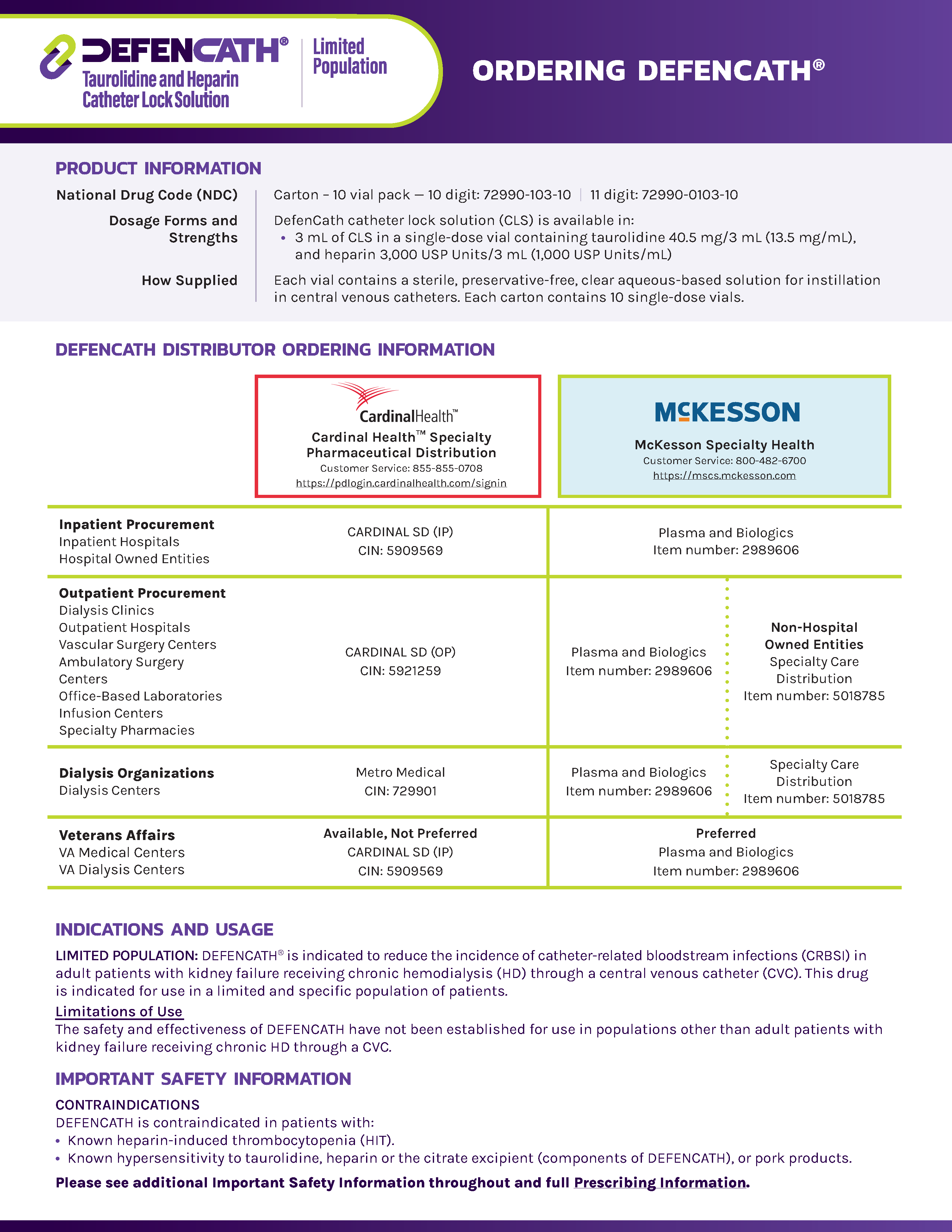
DefenCath Product Ordering Sheet
Download this resource to help navigate the product ordering process.
DownloadCRBSI = catheter-related bloodstream infection.
Reference: 1. DefenCath (taurolidine and heparin) [prescribing information]. Berkeley Heights, NJ: CorMedix Inc; 2023.